You may have to adjust downward using one of your initial serial dilutions so that the counts per small square are in the 5 to 15 cell range. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply.

Absorption Spectroscopy
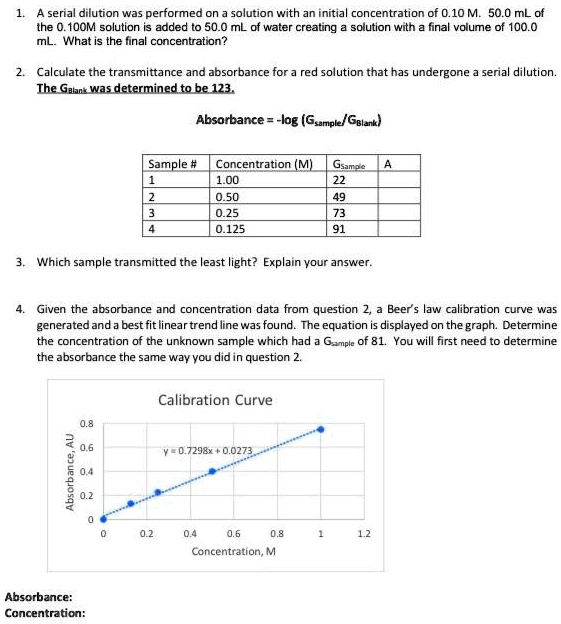
Solved Serial Dilution Was Performed On Solution With An Initial Concentration Of O 10 M 50 0 Ml Of The 0 1oom Solution Is Added To 50 0 Ml Of Water Creating Solution With Final Volume Of

A Uv Vis Absorption Spectra Of The Mos 2 Nanosheets Exfoliated In 10 Download Scientific Diagram
Many mollicutes strains die.
How to get the initial absorbance after dilution. The next sample is made from the previous dilution and the dilution factor is often kept constant. The absorption spectra of pH6-pH13 samples show maximum absorbance at 490 nm wavelength. This process continues through the last tube negative nine resulting in a final dilution factor of 11 billion.
A logarithmic measure of the amount of light that is absorbed when passing through a substance. Thus an automatic burette with a nitrogen atmosphere could be a choice to minimize the loss of free radical activity Blois 1958. After paying the order is assigned to the most qualified writer in that field.
This is done using a. An assay is an investigative analytic procedure in laboratory medicine mining pharmacology environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence amount or functional activity of a target entity. The dilution factor or the dilution is the initial volume divided by the final volume.
A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. The writer researches and then submits your. This details will be used by our support team to contact you.
Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. The analyte can be a drug biochemical substance chemical element or compound or cell in an organism or organic sample. If you have 10 µl of a sample and are diluting it 100 times and then taking in 5 µl from that diluted sample into the well your dilution factor would be 100.
Most hemoglobin derivatives oxyhemoglobin methemoglobin and carboxyhemoglobin but not sulfhemoglobin are converted to HiCN and therefore measured by this method. Thus care must be taken when making the initial solution. The optical absorbance spectra are recorded from 200 to 800 nm to determine the optical energy band gap of the pH6-pH13 samples Fig.
In serial dilutions you multiply the dilution factors for each step. This dilution series is important for titering purposes as well as to keep the culture in varying stages of growth. Measurement of the Product Concentration.
At day 90 after onset of symptoms or initial RT-PCR detection in asymptomatic infections it took 69 87 and 31 days for PRNT 50 antibody titres to decrease by half T 12 in severe mild and. In this case dilution would be 1 since you are taking pure undiluted 5 µl of the sample into the wells and the volume will be 0005 ml. Calculate the new concentration in mol L-1 molarity if enough water is added to 10000 mL of 025 mol L-1 sodium chloride solution to make up 15 L.
DF V_iV_f For example if you add a 1 mL sample to 9 mL of diluent to get 10 mL of solution. The absorbance line becomes almost plateaus between 400 and 490 nm. Calculate the initial concentrations of Fe3 and SCN in each test tube 1 - 5.
Time to get a line of slope -k. After filling out the order form you fill in the sign up details. The stock solution of DPPH slowly deteriorates.
C2 c1V1 V. The initial DPPH concentration should give absorbance values less than 10 50 to 100 μM. For second-order reactions graph the reciprocal of concentration vs.
DILUTION Red Colonies White Colonies Total Number 10-6 10-7 10-8 B. Spectrophotometry uses photometers known as spectrophotometers that can measure the intensity of a light beam at different wavelengthsAlthough spectrophotometry is most commonly applied to. Denaturation Final A 260 - Blank A 260 Initial A 260 - 1 200 Where Initial and Final A260 are the absorbance of the DNA at 260 nm before and after any denaturation treatments respectively.
After this 100 microliters of each dilution is plated. Calculate concentration of solution after dilution. You can now pay for your order.
Absorbance is a number that measures the attenuation of the transmitted light power in a solution. You may have to adjust downward using one of your initial serial dilutions so that the counts per small square are in the 5 to 15 cell range. Multiply the initial volume by the ratio of initial and final concentrations diminished by 1.
Relates the attenuation of light to the properties of the material through which the light is traveling. How to calculate the volume to be added in order to reach a specified concentration after the dilution. The dilution is the initial sample dilution.
Then 1mL of this solution is transferred to the next tube negative two resulting in a 1100 dilution factor. The disadvantage is that any errors in solution makingpipetting massing etcget propagated as more solutions are made. The assay in Figure 1 is linear up to optical density OD 25 and a dilution factor of 002 150 dilution of enzyme would be ideal for assay work as it gives a large signal 15 and lies in the middle of the linear range.
After 1 min 05 ml reaction mixture taken and added to 05 ml stop solution similarly after 23456 minute 05 ml sample taken and added to. This is just a dilution calculation. Resulting in a 110 dilution factor.
Get 247 customer support help when you place a homework help service order with us. Dilution used the original number of viable cells can be calculated. Even though the reaction appears to go instantaneously upon mixing the reactants the initial concentrations in the reaction table are those after dilution has been taken into consideration but before any reaction occurs.
We accept payment through PayPal and debit or credit cards. This is done using a. The advantage is that only one initial solution is needed.
Take your solutions to a Vernier colorimeter to measure the absorbance of the FeSCN ion-complex. The total reaction mixture of 3 ml contained 10 micro liter of enzyme extract and reaction was started after adding H2O2 and the absorbance was taken at an interval of 30 s for 3 minutes. Three variables Initial A 260 Final A 260 and Blank A 260 of the DNA were measured and implemented into Equation 1.
The term initial concentration can be confusing. Perform a serial dilution by transferring 05 mL from the first tube to a tube with 45 mL and then 05 mL from the second tube to a third tube etc. Absorbance of the diluted sample at 540 nm is compared with absorbance at the same wavelength of a standard HiCN solution whose equivalent hemoglobin concentration is known.
After that absorbance starts to go down.
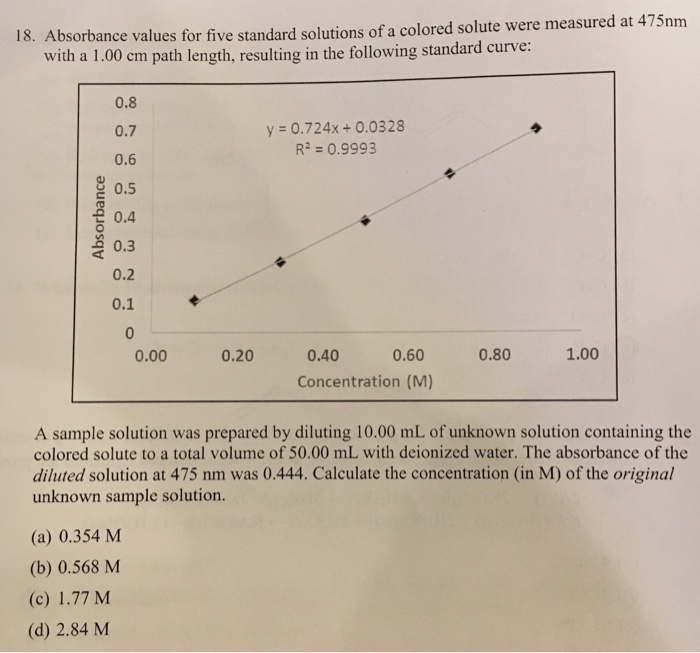
Solved 18 Absorbance Values For Five Standard Solutions Of Chegg Com
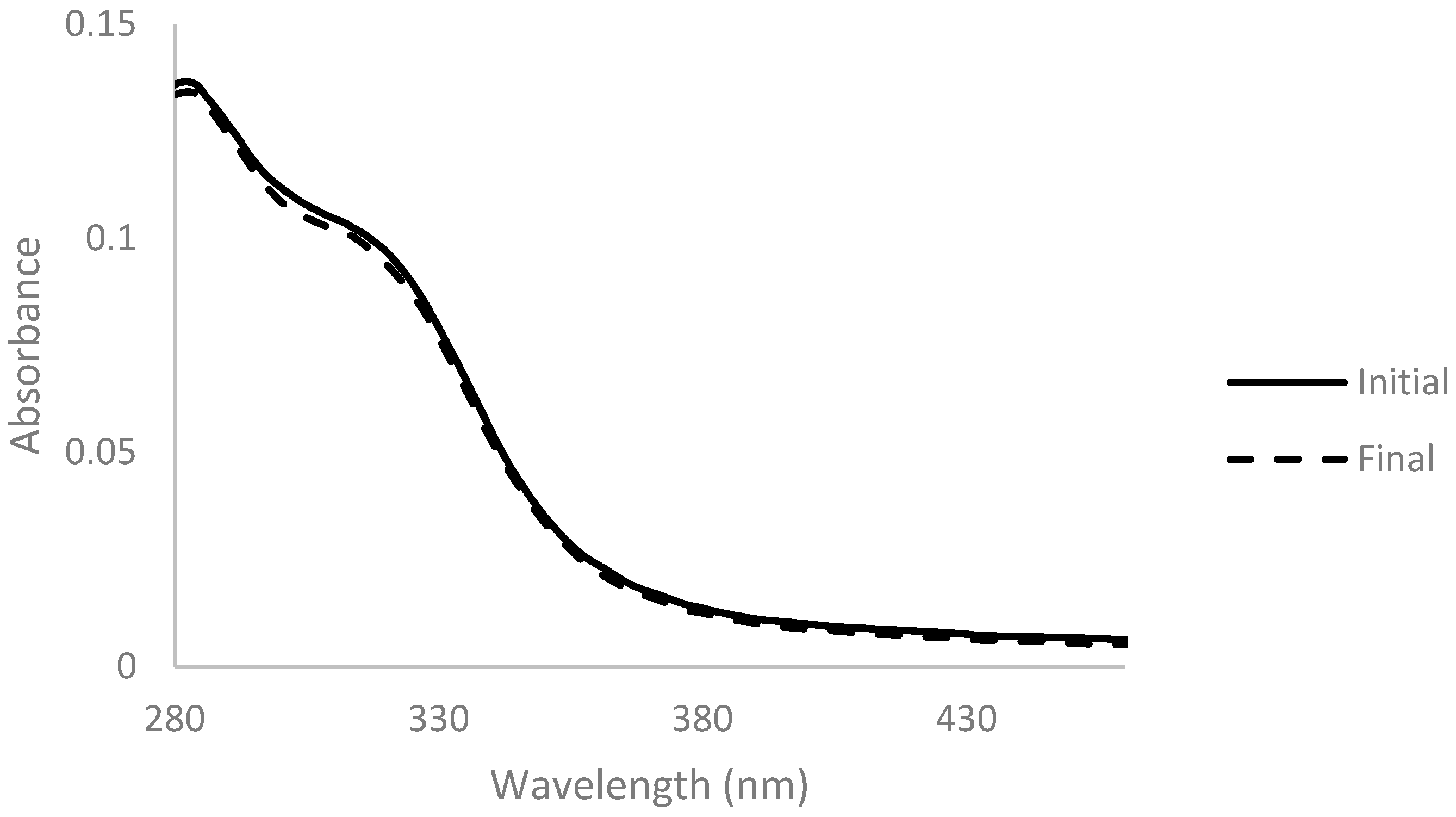
Membranes Free Full Text Decolorization Of A Corn Fiber Arabinoxylan Extract And Formulation Of Biodegradable Films For Food Packaging Html
Analytical Chemistry Uv Visible Spectroscopy
Dilution

Calculating Concentration Using The Beer Lambert Law Worked Example Video Khan Academy
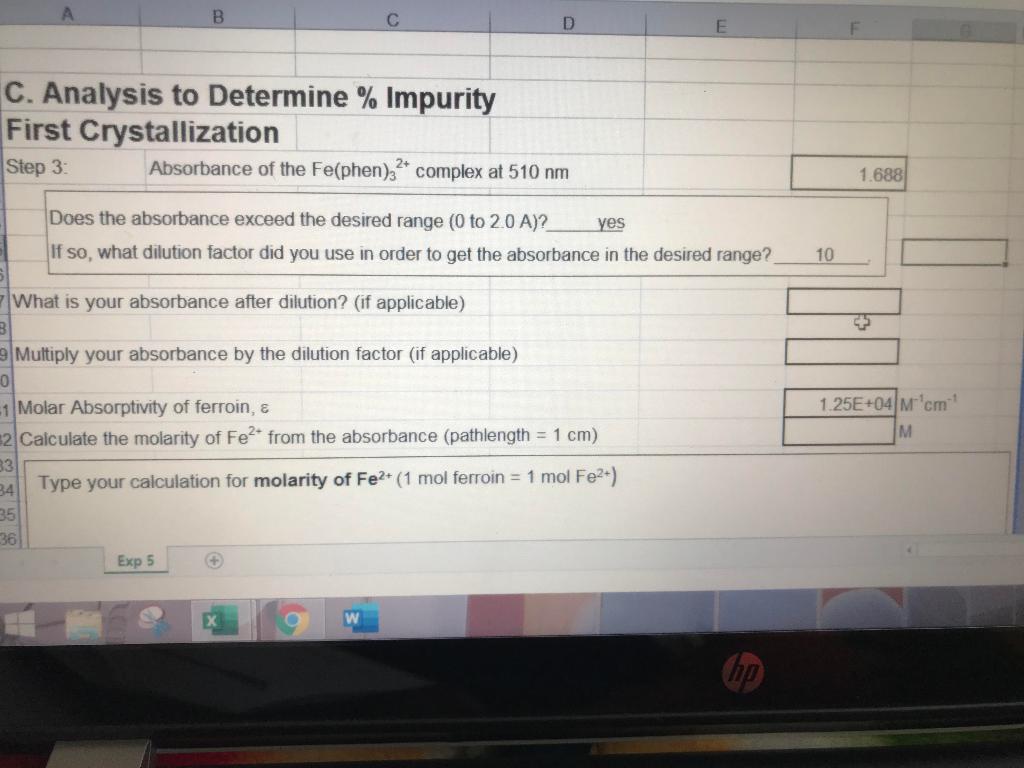
Solved How To Calculate What Is Your Absorbance After Chegg Com

Absorbance Vs Dilution Factor Scatter Chart Made By Bigkendog Plotly

Uv Vis Spectroscopy Purity Ratios The Bumbling Biochemist